I-125 Tumor Localization Seed Source
Product Description
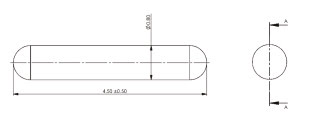
The I-125 source consists of a laser-welded titanium capsule containing iodine-125 adsorbed on a silver rod that acts as an x-ray detectable marker.

Propose Of The Use

Target User
Phisical Properties
Iodine-125 has a half-life of 59.4 days and decays due to the electron capture by the release of characteristic photons and electrons.
The major photon emissions are 27.2 keV, 27.5 keV, 31.0 keV and 35.5 keV with an average energy of 28.5 keV.
Table 1 indicates the decay chart of I-125 seeds.

Titanium tube properties: Grade 1 (It is not classified as implant class, as it will remain in the body for less than 29 days)
Tumor Marking Needle: 7 cm, 10 cm, 12 cm and 15 cm in length (should be selected according to breast type and size).
Needle Stopper: It is the barrier placed between the stylet and the tumor marking needle in order to prevent the I-125 seed source
placed in the tumor marking needle from unintentionally leaving the tumor marking needle
Target Patient Population
| CODE | CODE DESCRIPTION | |
|---|---|---|
| I125S | Product Code (I-125 Seed Source) | In the Product Serial Number, the "I125S" code is a fixed code describing the product. |
| A | Needle Length (cm) | The "A" coding indicates the length of the tumor marking needle in the product component. |
| B | Activity (MBq) | The "B" coding indicates the activity of the product. |
| C | Production Year (Last Two Digits) | The "C" coding refers to the last two digits of the year in which the serial numbered product was produced. |
| D | Product Number | The "D" coding is the sequence number that identifies the product produced in the product serial number. |
List Of Products
| Model No | Description | Activity | Sterility |
|---|---|---|---|
| I125S-7-2 | I-125 Loose Source(Single) in 18 Gauge x 7 cm Needle | 2 MBq | Sterile |
| I125S-7-9 | I-125 Loose Source(Single) in 18 Gauge x 7 cm Needle | 9 MBq | Sterile |
| I125S-10-2 | I-125 Loose Source(Single) in 18 Gauge x 10 cm Needle | 2 MBq | Sterile |
| I125S-10-9 | I-125 Loose Source(Single) in 18 Gauge x 10 cm Needle | 9 MBq | Sterile |
| I125S-12-2 | I-125 Loose Source(Single) in 18 Gauge x 12 cm Needle | 2 MBq | Sterile |
| I125S-12-9 | I-125 Loose Source(Single) in 18 Gauge x 12 cm Needle | 9 MBq | Sterile |
| I125S-15-2 | I-125 Loose Source(Single) in 18 Gauge x 15 cm Needle | 2 MBq | Sterile |
| I125S-15-9 | I-125 Loose Source(Single) in 18 Gauge x 15 cm Needle | 9 MBq | Sterile |
USAGE
Sterilization
Localization Dosage and Usage
Calibration
Storage Conditions
Temperature: 5 C to 30 C
Relative Humidity: 30% RH to 70% RH
Absolute Pressure: 100.0 kPa (14.504 psi, 0.987 atm)
Instructions for Safe Use
Precautions
Radiation Protection and Transport
Half Value Thickness-Lead: 0.025 mm
Half Value Thickness-Texture: 20 mm
With a thin lead film, the dose rate can be decreased by 99.9%. (0.25 mm or 0.01 inch). Less dosage exposure to healthcare personnel and patients is ensured when used with I-125 preservatives. I-125 seed sources can be used only by individuals trained in the safe use and handling of radioisotopes by a competent government authority.
Direct contact with I-125 seed sources should be prevented. The use of tweezers is recommended. While operating with sources, appropriate measures should be taken. Personnel monitoring is needed. For the monitoring of hand and whole body exposure, dose measuring instruments such as personal dosimeters should be used. All practical measures should be taken during the preparation stage and during the implantation of the source to ensure that exposure is kept relatively low. Measures such as reducing exposure time, increasing distances, proper preparation of administrative procedures and the use of preservatives should be considered in order to maintain the target time.
Leak Test
*For further details, please contact us to recieve IFU in your language.